Rising to the challenge
Timeline of Covid-19 at Vienna BioCenter (left) and in Austria (right)
With the arrival of SARS-CoV-2 in Europe in early 2020, scientists at the Vienna BioCenter quickly recognised the potential of basic research equipment and expertise for the development of innovative testing approaches. Within weeks, initiatives sprung up that soon led to important discoveries and tangible approaches for improving the monitoring of the virus.
December 2021
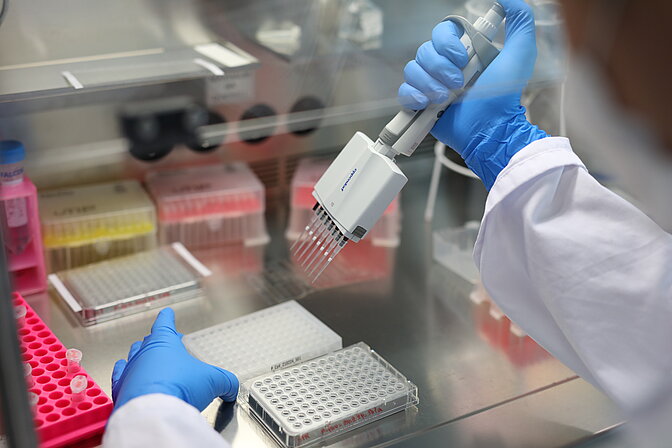
The original mission of in-house COVID testing was accomplished and testing on Vienna BioCenter campus is continued via 'Alles gurgelt'. Read more...
November / December 2021
3G mandatory at workplace, 2G rule applies everywhere else (start of lockdown for unvaccinated persons)
European Medicins Agency approves BioNTech's vaccine for children 5 years and older
Country-wide hard lockdown from Nov 22 to Dec 12
First case of new variant 'Omikron' in Austria
15 million vaccine doses administered, 3 million people received 3rd vaccination ('booster')
October / November 2021

Valneva reports Positive Phase 3 Results for Inactivated, Adjuvanted COVID-19 Vaccine Candidate VLA2001
Valneva signed an Advance Purchase Agreement (APA) with the European Commission (EC) to supply up to 60 million doses of its inactivated COVID-19 vaccine candidate, VLA2001, over two years.
Valneva confirmed that the European Medicines Agency (EMA) has started a rolling review of VLA2001.
September / October 2021
New tightening measures according to the federal government's step-by-step plan (FFP2 masks become mandatory indoors, 3G rule applies)
August 2021

Minister of Health W. Mückstein visits Vienna BioCenter. Through presentations and a campus tour, the contributions of Vienna BioCenter scientists in fighting the Covid-19 pandemic were showcased.
97.4 percent of approx. 800 employees at the campus' non-university research institutes vaccinated
May to July 2021
Country-wide opening steps (incl gastronomy, tourism, sports)
European Medicins Agency approves BioNTech's vaccine for children 12 years and older
Delta variant becomes most common variant in Austria.
5 million vaccine doses administered in Austria
May 2021
Ulrich Elling, Luisa Cochella, and others publish the method "SARSeq" in the journal Nature Communications. It could be adapted to many more pathogens, according to the scientists. Read more...
April 2021
3 million vaccine doses administered in Austria
Partial lockdown in eastern Austria ('Osterruhe')
Wolfgang Mückstein appointed Minister of Health after Rudolf Anschober's resignation
April 2021

Valneva initiates phase 3 clinical trial for its inactivated, adjuvanted COVID-19 vaccine candidate, VLA2001
March 2021
1 million vaccine doses administered in Austria
Start of 'Alles gurgelt' campaign in Vienna (free PCR self-sampling tests)
January / February 2021

Non-profit reagent kits for performing RT-LAMP and bead-LAMP are provided by the VDRC team at the Vienna BioCenter Core Facilities via see https://www.rtlamp.org/kits/
First Next Generation Sequencing run with SARSeq-S-tiling detects a cluster of B.1.351 variant (originally from South Africa) in Tyrol
Contract with AGES is signed for SARSeq-team to sequence a minimum of 1,000 positive SARS-CoV-2 samples per week in order to monitor variant spread and to identify new mutations
Valneva announces production start of its inactivated, adjuvanted COVID-19 vaccine candidate in parallel to the ongoing clinical studies
Dec 2020 - Jan 2021
Third hard lockdown in effect from Dec 26 to Jan 24, later extended until Feb 7
First person in Austria vaccinated, Start of country-wide vaccination campaign
December 2020

Valneva announces the initiation of a Phase 1/2 clinical study for its inactivated, adjuvanted COVID-19 vaccine candidate "VLA2001"
First variants of concern containing N501Y identified by the SARSeq team using Sanger sequencing and reported to AGES
Mid November 2020
Record number of reported new infections (9.209)
County-wide hard lockdown starting Nov 17 (all shops except basic services closed, 24h curfew)
October / November 2020

RT-LAMP website with complete protocols goes online: www.rtlamp.org steered by Pauli/Brennecke; European patent filed for Elling/Cochella SARSeq
SARSeq allowing high throughput testing of SARS-CoV-2 and other viruses is published as preprint on medRxiv
In-house testing at Vienna BioCenter is expanded to include VBC Companies
October 2020
Austrian Agency for Health and Food Safety (AGES) endorses RT-LAMP as fast, cheap and reliable testing method and viable complement to PCR tests
ÖQUASTA approval for SARSeq and RT-LAMP
September 2020
Sample processing and analysis moved to VCDI screening lab run by Vienna Biocenter Core Facilties
New test-kits and sampling logistics are rolled out under the responsibility of Johanna Trupke
Testing at Vienna BioCenter is expanded to include Max Perutz Labs and DOME
September 2020
Corona “traffic light” introduced by Austrian government
Press Conference: Heinz Faßmann and Peter Hacker present mobile testing initiative for schools in Vienna: gargling method, analysis at Vienna BioCenter
Mid May to August 2020
1.1 million Euro granted to IMBA by the European Commission as part of the MAD-CoV 2 project, funding further COVID19 research
Start of antibody-testing for IMP/IMBA/GMI/VBCF employees
VCDI releases complete SOP for routine SARS-CoV-2 monitoring
VCDI initiates large-scale monitoring project at Austrian schools using gargling in combination with pooling and RT-qPCR
May 2020
Restaurants reopen in Austria, border to Germany and Switzerland reopens
Gradual reopening of Austrian schools; second Austria-wide prevalence study
2,000 confirmed active cases in Austria
Second half of April 2020
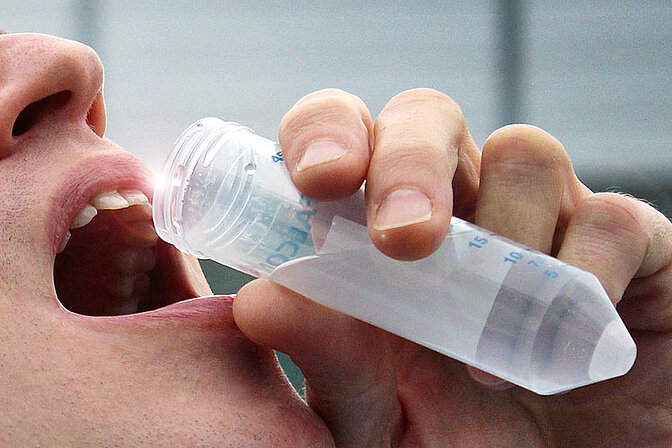
Gradual reopening of the institutes with shift plans and safety measures in place
Start of in-house COVID-19 testing at IMP/IMBA/GMI/VBCF (regular RT-qPCR tests on pharyngeal lavage samples) - voluntary and anonymous
14 April 2020
Partial lockdown in Austria eased gradually
Early April 2020
Several research institutions join forces to form the “Vienna COVID-19 Diagnostics Initiative (VCDI)”, later renamed into “Vienna COVID-19 Detection Initiative”
IMBA receives WWTF funding for its involvement in three different COVID19 projects, as part of the WWTF’s COVID-19 Rapid Response Funding
Standard Operating Procedure (SOP) shared with employees to allow regular RT-qPCR testing for IMP/IMBA/GMI/VBCF staff
Meinrad Busslinger initiates IgM/IgG antibody testing for employees
16 March 2020
Partial lockdown in Austria, schools and universities close
Mid March 2020

Andrea Pauli (IMP) and Julius Brennecke (IMBA) & team start a project on fast and easy SARS-CoV-2 detection using RT-LAMP
Johannes Zuber (IMP) and team start testing alternative sampling-methods (gargling)
Luisa Cochella (IMP), Ulrich Elling (IMBA), Alexander Stark (IMP) and Ramesh Yelagandula (IMBA) start a project on NGS-based COVID-19 testing
IMP/IMBA/GMI/VBCF issue strict limitations and safety rules, operations are reduced to a minimum, all but essential staff is working from home
11 March 2020
WHO declares pandemic
10 March 2020
Safety regulations at the institutes are tightened, events with more than 100 participants cancelled
25 February 2020
First COVID-19 infections detected in Austria




